Executing Baseline Algorithms¶
In this section we introduce the baselines available in this pakcage. To execute one of then in the databases available just run the following command:
$ bob bio pipeline simple [DATABASE_NAME] [BASELINE]
Note
Both, [DATABASE_NAME] and [BASELINE] can be either python resources or python files.
Please, refer to bob.bio.base for more information.
Repeated Line-Tracking with Miura Matching¶
Detailed description at Repeated Line Tracking and Miura Matching.
To run the baseline on the VERA fingervein database, using the Nom
protocol (deprecated), do the following:
$ bob bio pipeline simple verafinger rlt -vv -c
Tip
If you have more processing cores on your local machine and don’t want to
submit your job for SGE execution, you can run it in parallel by adding the options -l local-parallel.
$ bob bio pipeline simple verafinger rlt -vv -c -l local-parallel
To run on the Idiap SGE grid use:
$ bob bio pipeline simple rlt -vv -c -l sge
This command line selects and runs the following implementations for the toolchain:
bob.bio.vein.resources.database.verafinger
As the tool runs, you’ll see printouts that show how it advances through preprocessing, feature extraction and matching. In a 4-core machine and using 4 parallel tasks, it takes around 4 hours to process this baseline with the current code implementation.
To complete the evaluation, run the command bellow, that will output the equal error rate (EER) and plot the detector error trade-off (DET) curve with the performance:
$ bob bio metrics <path-to>/verafinger/rlt/Nom/nonorm/scores-dev --no-evaluation
[Min. criterion: EER ] Threshold on Development set `scores-dev`: 0.31835292
====== ========================
None Development scores-dev
====== ========================
FtA 0.0%
FMR 23.6% (11388/48180)
FNMR 23.6% (52/220)
FAR 23.6%
FRR 23.6%
HTER 23.6%
====== ========================
Maximum Curvature with Miura Matching¶
Detailed description at Maximum Curvature and Miura Matching.
To run the baseline on the VERA fingervein database, using the Nom
protocol like above, do the following:
$ bob bio pipeline simple verafinger mc -vv -c
This command line selects and runs the following implementations for the toolchain:
bob.bio.vein.resources.database.verafinger
In a 4-core machine and using 4 parallel tasks, it takes around 1 hour and 40 minutes to process this baseline with the current code implementation. Results we obtained:
$ bob bio metrics <path-to>/verafinger/mc/Nom/nonorm/scores-dev --no-evaluation
[Min. criterion: EER ] Threshold on Development set `scores-dev`: 7.372830e-02
====== ========================
None Development scores-dev
====== ========================
FtA 0.0%
FMR 4.4% (2116/48180)
FNMR 4.5% (10/220)
FAR 4.4%
FRR 4.5%
HTER 4.5%
====== ========================
Wide Line Detector with Miura Matching¶
You can find the description of this method on the paper from Huang et al. [HDLTL10].
To run the baseline on the VERA fingervein database, using the Nom
protocol like above, do the following:
$ bob bio pipeline simple verafinger wld -vv -c
This command line selects and runs the following implementations for the toolchain:
bob.bio.vein.resources.database.verafinger
In a 4-core machine and using 4 parallel tasks, it takes only around 5 minutes minutes to process this baseline with the current code implementation.Results we obtained:
$ bob bio metrics <path-to>/verafinger/wld/Nom/nonorm/scores-dev --no-evaluation
[Min. criterion: EER ] Threshold on Development set `scores-dev`: 2.402707e-01
====== ========================
None Development scores-dev
====== ========================
FtA 0.0%
FMR 9.8% (4726/48180)
FNMR 10.0% (22/220)
FAR 9.8%
FRR 10.0%
HTER 9.9%
Results for other Baselines¶
This package may generate results for other combinations of protocols and databases. Here is a summary table for some variants (results expressed correspond to the the equal-error rate on the development set, in percentage):
Toolchain |
Vera Finger |
UTFVP |
|||
|---|---|---|---|---|---|
Feature Extractor |
Full |
B |
Nom |
1vsall |
nom |
Repeated Line Tracking |
14.6 |
13.4 |
23.6 |
3.4 |
1.4 |
Wide Line Detector |
5.8 |
5.6 |
9.9 |
2.8 |
1.9 |
Maximum Curvature |
2.5 |
1.4 |
4.5 |
0.9 |
0.4 |
In a machine with 48 cores, running these baselines took the following time (hh:mm):
Toolchain |
Vera Finger |
UTFVP |
|||
|---|---|---|---|---|---|
Feature Extractor |
Full |
B |
Nom |
1vsall |
nom |
Repeated Line Tracking |
01:16 |
00:23 |
00:23 |
12:44 |
00:35 |
Wide Line Detector |
00:07 |
00:01 |
00:01 |
02:25 |
00:05 |
Maximum Curvature |
03:28 |
00:54 |
00:59 |
58:34 |
01:48 |
Modifying Baseline Experiments¶
It is fairly easy to modify baseline experiments available in this package. To do so, you must copy the configuration files for the given baseline you want to modify, edit them to make the desired changes and run the experiment again.
For example, suppose you’d like to change the protocol on the Vera Fingervein
database and use the protocol full instead of the default protocol nom.
First, you identify where the configuration file sits:
$ resources.py -tc -p bob.bio.vein
- bob.bio.vein X.Y.Z @ /path/to/bob.bio.vein:
+ mc --> bob.bio.vein.configurations.maximum_curvature
+ parallel --> bob.bio.vein.configurations.parallel
+ rlt --> bob.bio.vein.configurations.repeated_line_tracking
+ utfvp --> bob.bio.vein.configurations.utfvp
+ verafinger --> bob.bio.vein.configurations.verafinger
+ wld --> bob.bio.vein.configurations.wide_line_detector
The listing above tells the verafinger configuration file sits on the
file /path/to/bob.bio.vein/bob/bio/vein/configurations/verafinger.py. In
order to modify it, make a local copy. For example:
$ cp /path/to/bob.bio.vein/bob/bio/vein/configurations/verafinger.py verafinger_full.py
$ # edit verafinger_full.py, change the value of "protocol" to "full"
Also, don’t forget to change all relative module imports (such as from
..database.verafinger import Database) to absolute imports (e.g. from
bob.bio.vein.database.verafinger import Database). This will make the
configuration file work irrespectively of its location w.r.t. bob.bio.vein.
The final version of the modified file could look like this:
from bob.bio.vein.database.verafinger import Database
database = Database(original_directory='/where/you/have/the/raw/files',
original_extension='.png', #don't change this
)
protocol = 'full'
Now, re-run the experiment using your modified database descriptor:
$ bob bio pipeline simple ./verafinger_full.py wld -vv -c
Notice we replace the use of the registered configuration file named
verafinger by the local file verafinger_full.py. This makes the program
verify.py take that into consideration instead of the original file.
Other Resources¶
This package contains other resources that can be used to evaluate different bits of the vein processing toolchain.
Region of Interest Goodness of Fit¶
Automatic region of interest (RoI) finding and cropping can be evaluated using
a couple of scripts available in this package. The program
bob_bio_vein_compare_rois.py compares two sets of preprocessed images
and masks, generated by different preprocessors (see
bob.bio.base.preprocessor.Preprocessor) and calculates a few
metrics to help you determine how both techniques compare. Normally, the
program is used to compare the result of automatic RoI to manually annoted
regions on the same images. To use it, just point it to the outputs of two
experiments representing the manually annotated regions and automatically
extracted ones. E.g.:
$ bob_bio_vein_compare_rois.py ~/verafinger/mc_annot/preprocessed ~/verafinger/mc/preprocessed
Jaccard index: 9.60e-01 +- 5.98e-02
Intersection ratio (m1): 9.79e-01 +- 5.81e-02
Intersection ratio of complement (m2): 1.96e-02 +- 1.53e-02
Values printed by the script correspond to the Jaccard index
(bob.bio.vein.preprocessor.utils.jaccard_index()), as well as the
intersection ratio between the manual and automatically generated masks
(bob.bio.vein.preprocessor.utils.intersect_ratio()) and the ratio to
the complement of the intersection with respect to the automatically generated
mask
(bob.bio.vein.preprocessor.utils.intersect_ratio_of_complement()). You
can use the option -n 5 to print the 5 worst cases according to each of the
metrics.
Pipeline Display¶
You can use the program bob_bio_vein_view_sample.py to display the images
after full processing using:
$ bob_bio_vein_view_sample.py --save=output-dir verafinger /path/to/processed/directory 030-M/030_L_1
$ # open output-dir
And you should be able to view images like these (example taken from the Vera fingervein database, using the automatic annotator and Maximum Curvature feature extractor):
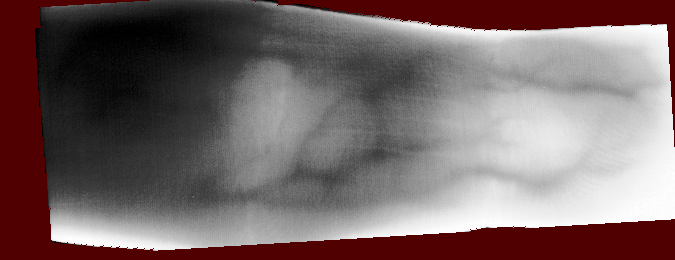
Fig. 1 Example RoI overlayed on finger vein image of the Vera fingervein database,
as produced by the script bob_bio_vein_view_sample.py.¶
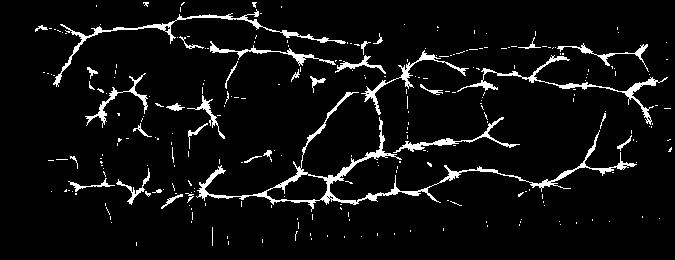
Fig. 2 Example of fingervein image from the Vera fingervein database, binarized by using Maximum Curvature, after pre-processing.¶